T021 · One-Hot Encoding¶
Developed in the CADD seminar 2020, Volkamer Lab, Charité/FU Berlin
Note: This talktorial is a part of TeachOpenCADD, a platform that aims to teach domain-specific skills and to provide pipeline templates as starting points for research projects.
Authors:
Sakshi Misra, CADD seminar 2020, Charité/FU Berlin
Talia B. Kimber, 2020, Volkamer lab, Charité
Yonghui Chen, 2020, Volkamer lab, Charité
Andrea Volkamer, 2020, Volkamer lab, Charité
Aim of this talktorial¶
The aim of the talktorial is to perform one-hot encoding of SMILES structures on a subset of the ChEMBL data set to gain a deeper understanding on the one-hot encoding concept and why it is useful as a pre-processing step in various machine learning algorithms.
Contents in Theory¶
Molecular data and representation
ChEMBL database
SMILES structures and rules
What is categorical data?
What is the problem with categorical data?
How to convert categorical data to numerical data?
The One-Hot Encoding (OHE) concept
Why using one-hot encoding?
Example of one-hot encoding
Advantages and disadvantages of one-hot encoding
Similar: Integer or label encoding
What is padding?
Further readings
Contents in Practical¶
Import necessary packages
Read the input data
Process the data
Double digit replacement
Compute longest (& shortest) SMILES
Python one-hot encoding implementation
One-hot encode (padding=True)
Visualization
Shortest SMILES
Longest SMILES
Supplementary material
Scikit learn implementation
Keras implementation
References¶
Theoretical background:
ChEMBL database: “The ChEMBL bioactivity database: an update.” (Nucleic acids research (2014), 42.D1, D1083-D1090)
Allen Chieng Hoon Choong, Nung Kion Lee, “Evaluation of Convolutionary Neural Networks Modeling of DNA Sequences using Ordinal versus one-hot Encoding Method”, bioRxiv, October 25, 2017.
Patricio Cerda, Gael Varoquaux, “Encoding high-cardinality string categorical variables”, arXiv:1907, 18 May 2020.
Blogpost: Jason Brownlee, How to One Hot Encode Sequence Data in Python, Machine Learning Mastery, accessed November 9th, 2020.
Blogpost: Krishna Kumar Mahto, One-Hot-Encoding, Multicollinearity and the Dummy Variable Trap, towardsdatascience, Available from one-hot-encoding-multicollinearity, accessed July 8th, 2019.
Blogpost: Chris, What is padding in a neural network?, archieved from MachineCurve, Padding
Packages and functions:
RDKit: Greg Landrum, RDKit Documentation, PDF, Release on 2019.09.1.
-
Scikit-learn: Machine Learning in Python, Pedregosa et al., JMLR 12, pp. 2825-2830, 2011.
Jiangang Hao, et al. “A Review of Scikit-learn Package in Python Programming Language.” Journal of Education and Behavioral Statistics Volume: 44 issue: 3 (2019), page(s): 348-361
Keras: Book chapter: “An Introduction to Deep Learning and Keras” in Learn Keras for Deep Neural Networks (2019), page(s):1-16.
smiles encoderfunction: Blogpost by iwatobipen, encode and decode SMILES strings , Wordpress, accessed November 9th, 2020
Theory¶
Molecular data and representation¶
ChEMBL database¶
ChEMBL is an open large-scale bioactivity database, containing molecules with drug-like properties.
The recent release (version 25) contains information extracted from more than \(72,000\) documents. In total, there are now more than \(1.8\) million compounds and \(15\) million bioactivity data points available.
It is maintained by European Bioinformatics Institute. Please refer to Talktorial T001 for more details.
SMILES structures and rules¶
SMILES (Simplified Molecular Input Line Entry System) notation is a chemical notation that allows a user to represent a chemical structure of a molecule in a linear way that can be read by the computer (see “Modern Aspects of the Smiles Rearrangement” (2017), Chemistry A European Journal, Volume23, Issue38, 8992-9008 for further information).
It contains a sequence of letters, numbers and characters that specify a molecule’s atoms, their connectivity, bond order and chirality.
Some SMILES specification rules
Atoms - are represented by their atomic symbols. Also metal atoms are represented with symbols in square bracket, e.g. Gold
[Au].Bonds - single, double and triple bonds are represented by symbols
-,=and#, respectively. Single bonds are the default and therefore do not need to be specified.Aromaticity - While atomic symbols are generally used in upper case, such as
C,O,SandN; to specify aromatic atoms lower case symbols are used instead, such asc,o,sandn. Sometimes implicit bonds in rings (alternating=and-) are also used to describe aromatic atoms such asC1=CC=CC=C1.Rings - SMILES allows a user to identify ring structures by using numbers to identify the opening and closing ring atom. For example, in
C1CCCCC1, the first carbon has a number “1” which connects by a single bond with the last carbon which also has a number “1”. The resulting structure is cyclohexane.Branches - are specified by enclosing them in parentheses, and can be nested or arranged. For example, 2-Propanol is represented by
CC(O)C.
What is categorical data?¶
Categorical data are variables that contain labels rather than numeric values. Some examples include:
A “pet” variable with the values: “dog” and “cat”.
A “color” variable with the values: “red”, “green” and “blue”.
A “place” variable with the values: “first”, “second” and “third”.
What is the problem with categorical data?¶
Machine learning consists of mathematical operations translated to a computer via low-level programming languages. Computers are brilliant when dealing with numbers. So we must somehow convert our input data to numbers. There are many machine learning algorithms which cannot operate on categorical data directly. Thus, categorical data must be converted to a numerical form, that all input and output variables are numeric (see Blogpost: Alakh Sethi, One-Hot Encoding vs. Label Encoding using Scikit-Learn , Analytics Vidya, accessed March 6th, 2020 for further information).
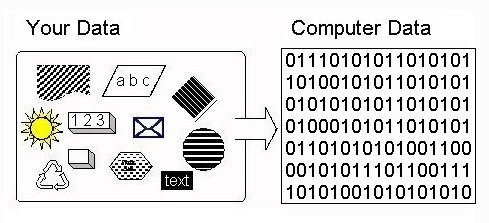
Figure 1: Displays the categorical encoding required for computers to understand the input. The figure comes from this blogpost.
How to convert categorical data to numerical data?¶
There are many ways to convert categorical values into numerical values. Each approach has its own positive and negative impact on the feature set. Hereby, two main methods will be the focus: One-hot encoding and Label encoding. Both of these encoders are part of the scikit-learn library (one of the most widely used Python libraries) and are used to convert text or categorical data into numerical data which the model expects and and can work with.
The One-Hot Encoding (OHE) concept¶
The one-hot encoding is a vector representation where all the elements of the vector are set to 0 except one, which has 1 as its value. For example, [0 0 0 1 0 0] is a one-hot vector. Simply put, one-hot encoding, also known as binary encoding, is a binary representation of categorical variables as binary vectors.
The figure shown below helps us gain an overall idea of the one-hot encoding concept.
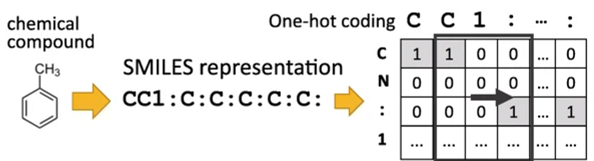
Figure 2 : Displays the one-hot encoding of the toluene molecule. Figure taken from the article BMC Bioinformatics. (2018), 19,526, more information can be found there.
Let us take a deeper look into the concept with the help of a simple example that will describe the basic concept of one-hot encoding, why it is useful and how one can approach it.
Why using one-hot encoding?¶
One-hot encoding allows the representation of categorical data to be more expressive. It is difficult for many machine learning algorithms to work with categorical data directly that’s why the label values which are categorical must be converted into numbers first as a preprocessing step. This is required for both input and output variables that are categorical.
We could also use an integer encoding directly. This may work for problems where there is a natural ordinal relationship between the categories, and in turn the integer values, such as labels for temperature “cold”, “warm”, and “hot”. There may be problems when there is no ordinal relationship and allowing the representation to lean on any such relationship by using integer encoding might be not useful to solve the problem. An example might be the labels “dog” and “cat”.
Example of one-hot encoding¶
Let us take a look at a very simple example to understand this concept. Assume we have the “color” variable which has three labels red , blue and green. All these labels must be converted into numeric form in order to work with our machine learning algorithm. This can be done by creating three new columns having all three labels and use 1 for the color of the respective label and 0 for the other colors as shown in Figure 4.
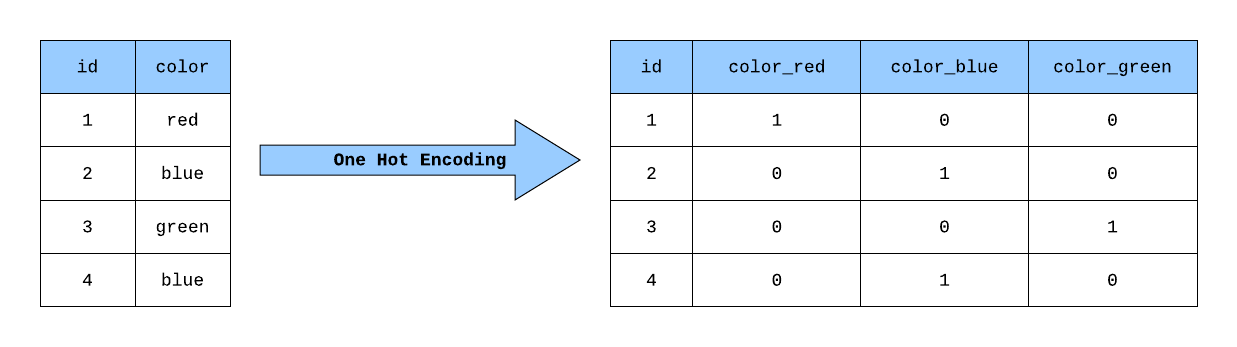
Figure 3 : The visual demonstration of one-hot encoding done on the variable “color”. Figure taken from the article: “Building a One Hot Encoding Layer with TensorFlow”, George Novack, towardsdatascience, more details can be found there.
Advantages and disadvantages of one-hot encoding¶
Advantages - If the cardinality (the number of categories) of the categorical features is low (relative to the amount of data), one-hot encoding will work best. - One-hot encoding has the advantage that the result is binary rather than ordinal and that everything sits in an orthogonal vector space.
Disadvantages - Increase in dimensionality, after adding several columns based on categorical variables which may result in an increase in the computational cost. - There is a high chance of multi-collinearity due to dummy variables (unique category added as a feature) which can affect the performance of the model. - One-hot encoding can result in increasing the sparsity of a data set (a sparse matrix is a matrix in which most of the elements are zero).
Similar: Integer or label encoding¶
Label Encoding, or integer encoding, is a popular encoding technique for handling categorical variables and is easily reversible. In this technique, each label is assigned a unique integer based on alphabetical ordering, so that machines can work with it properly. Machine learning algorithms can then decide in a better way on how labels must be operated on. It is an important preprocessing step for structured data sets in supervised learning.
Example of integer encoding
Let us use a similar example as above: We have a color variable and we can assign red as 0, green as 1, and blue as 2 as shown in Figure 5.
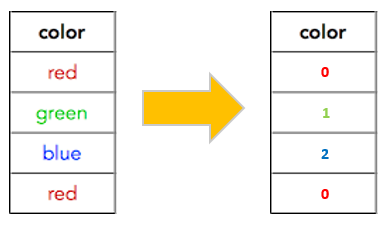
Figure 5 : The visual demonstration of label encoding done on the variable “color”. Figure taken from the article: “Know about Categorical Encoding, even New Ones!”, Ahmed Othmen, towardsdatascience, more details can be found there.
Difference between label and one-hot encoding
There is not much difference between these two encoding techniques, it mainly depends on the type of data and model used. For example, if we have categorical features which are not ordinal (dog or cat) then we can use one-hot encoding. Label encoding works best with ordinal data like good=0, better=1, best=2. Also when there are many categorical variables then it might be good to choose label encoding just to avoid high memory consumption and sparsity.
What is padding?¶
Padding is used to add zeros to the resulted one-hot encoded matrix. There are different types of padding, we chose to performed zero padding in here. For more details, please refer to this Article.
Why is it performed?
Padding is performed to make the dimensions of the matrix equal - or to preserve the height and the width - and to not have to worry too much about tensor dimensions when used as an input for the deep learning models.
How is it performed?
Padding can be performed by using the numpy.pad function which takes several parameters like the array which needs to be padded, pad_width which is number of values added to the edges of each axis and mode which by default is “constant”.
In this talktorial, padding is performed
implicitly: when applying one-hot encoding using a python implementation on the preprocessed data, where we have given the maximum length of the string as the parameter so that all the resulting one-hot encoded matrices are of the same dimension
explicitly: More info on this can be found in the supplementary section, where we apply one-hot encoding using keras and scikit-learn implementations.
Further readings¶
This section lists some resources for further reading:
What is one-hot encoding and when is it used in data science?
Categorical encoding using Label-Encoding and One-Hot-Encoder
Hirohara, M., Saito, Y., Koda, Y. et al. Convolutional neural network based on SMILES representation of compounds for detecting chemical motif. BMC Bioinformatics 19, 526 (2018)
Practical¶
Import necessary packages¶
[1]:
from pathlib import Path
from warnings import filterwarnings
import pandas as pd
import numpy as np
from rdkit import Chem
from rdkit.Chem import Draw, PandasTools
from sklearn.preprocessing import LabelEncoder
from sklearn.preprocessing import OneHotEncoder
from tensorflow import keras
from tensorflow.keras.utils import to_categorical
from tensorflow.keras.preprocessing.text import Tokenizer
import matplotlib.pyplot as plt
# Silence some expected warnings
filterwarnings("ignore")
[2]:
# Set path to this notebook
HERE = Path(_dh[-1])
DATA = HERE / "data"
Read the input data¶
Using the Pandas library, we first load the subset of the ChEMBL data set and draw the molecules using the rdkit.draw function. We then preprocess the data and apply our one-hot encoding python implementation.
Let’s load the data and quickly analyze its column values and check if there are any missing values:
[3]:
df = pd.read_csv(DATA / "CHEMBL25_activities_EGFR.csv", index_col=0).reset_index(drop=True)
print(f"Shape of dataframe: {df.shape}\n")
# NBVAL_CHECK_OUTPUT
Shape of dataframe: (3905, 5)
Check the dimension and missing value of the data
[4]:
df.info()
<class 'pandas.core.frame.DataFrame'>
RangeIndex: 3905 entries, 0 to 3904
Data columns (total 5 columns):
# Column Non-Null Count Dtype
--- ------ -------------- -----
0 chembl_id 3905 non-null object
1 IC50 3905 non-null float64
2 units 3905 non-null object
3 canonical_smiles 3905 non-null object
4 pIC50 3905 non-null float64
dtypes: float64(2), object(3)
memory usage: 152.7+ KB
Look at the first 3 rows
[5]:
df.head(3)
# NBVAL_CHECK_OUTPUT
[5]:
| chembl_id | IC50 | units | canonical_smiles | pIC50 | |
|---|---|---|---|---|---|
| 0 | CHEMBL207869 | 77.0 | nM | Clc1c(OCc2cc(F)ccc2)ccc(Nc2c(C#Cc3ncccn3)cncn2)c1 | 7.113509 |
| 1 | CHEMBL3940060 | 330.0 | nM | ClCC(=O)OCCN1C(=O)Oc2c1cc1c(Nc3cc(Cl)c(F)cc3)n... | 6.481486 |
| 2 | CHEMBL3678951 | 1.0 | nM | FC(F)(F)c1cc(Nc2n(C(C)C)c3nc(Nc4ccc(N5CC[NH+](... | 9.000000 |
Select the columns which are necessary for our study
[6]:
df = df[["chembl_id", "canonical_smiles"]]
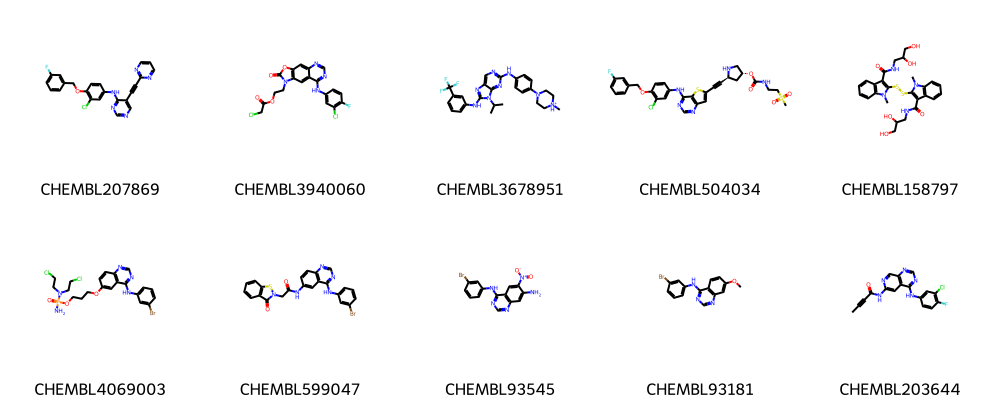
We can visualize the molecules with their ChEMBL ID using pandas tools and the draw method as shown below.
[7]:
# Using PandasTools and the respective Draw method
PandasTools.AddMoleculeColumnToFrame(df, smilesCol="canonical_smiles", molCol="Mol2D")
Draw.MolsToGridImage(list(df.Mol2D[0:10]), legends=list(df.chembl_id[0:20]), molsPerRow=5)
[7]:
Process the data¶
SMILES preprocessing: Since in SMILES representation, atoms can be described by one or two characters (depending on the periodic element they represent) while the machine will read the input position-wise, we convert the SMILES in a chemical-aware representation.
First, we search for all unique characters present in the current data set, which allows us to remove the characters which are not present in the current data.
Second, we search for all double-character elements in our SMILES data set by comparing the atoms present in our strings with all the possible elements present in the periodic table and replacing all the two alphabetic elements by artificially selected characters, for example changing
CltoL.
For padding:
SMILES strings have unequal dimension since their string length differ. For machine learning application, having equal dimension throughout the data set is required. In order to achieve this, we can first search for the SMILES string with the maximum length (e.g. len() method) and pass it as an argument in our function for all the strings.
Double digit replacement¶
[8]:
def assess_two_letter_elements(df):
"""
Find the two letter elements in dataframe.
Parameters
----------
df : pandas.DataFrame
Dataframe which requires preprocessing.
Returns
-------
two_letter_elements : list
List with found two letter elements
"""
# Search for unique characters in SMILES strings
unique_chars = set(df.canonical_smiles.apply(list).sum())
# Get upper and lower case letters only
upper_chars = []
lower_chars = []
for entry in unique_chars:
if entry.isalpha():
if entry.isupper():
upper_chars.append(entry)
elif entry.islower():
lower_chars.append(entry)
print(f"Upper letter characters {sorted(upper_chars)}")
print(f"Lower letter characters {sorted(lower_chars)}")
# List of all possible periodic elements
periodic_elements = [
"Ac",
"Al",
"Am",
"Sb",
"Ar",
"As",
"At",
"Ba",
"Bk",
"Be",
"Bi",
"Bh",
"B",
"Br",
"Cd",
"Ca",
"Cf",
"C",
"Ce",
"Cs",
"Cl",
"Cr",
"Co",
"Cn",
"Cu",
"Cm",
"Ds",
"Db",
"Dy",
"Es",
"Er",
"Eu",
"Fm",
"Fl",
"F",
"Fr",
"Gd",
"Ga",
"Ge",
"Au",
"Hf",
"Hs",
"He",
"Ho",
"H",
"In",
"I",
"Ir",
"Fe",
"Kr",
"La",
"Lr",
"Pb",
"Li",
"Lv",
"Lu",
"Mg",
"Mn",
"Mt",
"Md",
"Hg",
"Mo",
"Mc",
"Nd",
"Ne",
"Np",
"Ni",
"Nh",
"Nb",
"N",
"No",
"Og",
"Os",
"O",
"Pd",
"P",
"Pt",
"Pu",
"Po",
"K",
"Pr",
"Pm",
"Pa",
"Ra",
"Rn",
"Re",
"Rh",
"Rg",
"Rb",
"Ru",
"Rf",
"Sm",
"Sc",
"Sg",
"Se",
"Si",
"Ag",
"Na",
"Sr",
"S",
"Ta",
"Tc",
"Te",
"Ts",
"Tb",
"Tl",
"Th",
"Tm",
"Sn",
"Ti",
"W",
"U",
"V",
"Xe",
"Yb",
"Y",
"Zn",
"Zr",
]
# The two_char_elements list contains all two letter elements
# which can be generated by all possible combination of upper x lower characters
# and are valid periodic elements.
two_char_elements = []
for upper in upper_chars:
for lower in lower_chars:
ch = upper + lower
if ch in periodic_elements:
two_char_elements.append(ch)
# This list is then reduced to the subset of two-letter elements
# that actually appear in the SMILES strings, specific to our data set.
two_char_elements_smiles = set()
for char in two_char_elements:
if df.canonical_smiles.str.contains(char).any():
two_char_elements_smiles.add(char)
return two_char_elements_smiles
[9]:
elements_found = assess_two_letter_elements(df)
print(f"\nTwo letter elements found in the data set: {sorted(elements_found)}")
# NBVAL_CHECK_OUTPUT
Upper letter characters ['B', 'C', 'F', 'H', 'I', 'N', 'O', 'P', 'S']
Lower letter characters ['c', 'e', 'l', 'n', 'o', 'r', 's']
Two letter elements found in the data set: ['Br', 'Cl', 'Cn', 'Sc', 'Se']
Based on this finding, we defined our own dictionary for replacement. Note that their are several shortcomings with this simple implementation that we (partially) manually address here:
We exclude
ScandCnfrom the replacement, since it is more likely that sulfurSand an aromatic carboncare contained in a molecule than scandiumSc, same for carbonCand an aromatic nitrogennover CoperniciumCn. Thus, only the elements chlorineCl, bromineBrand seleniumSeare replaced.In isomeric SMILES
@and@@are used to describe enantiomers, thus we also need to replace the latter by a one letter code.If you are working wit a different data set, you may want to adapt the below mapping dictionary.
This resulted in the following dictionary to replace the two letter elements found in this data set
[10]:
replace_dict = {"Cl": "L", "Br": "R", "Se": "X", "@@": "Z"}
Based on this dictionary, we define a function to create the preprocessed data.
[11]:
def preprocessing_data(df, replacement):
"""
Preprocess the SMILES structures in a data set.
Parameters
----------
df : pandas.DataFrame
Dataframe which requires preprocessing.
replacement : dict
Dictionary with mapping for replacement.
Returns
-------
df : pandas.DataFrame
Dataframe with new processed canonical SMILES column.
unique_char : list
List with unique characters present in SMILES.
"""
# Print warning if the data set has a 'Sc' element
if df.canonical_smiles.str.contains("Sc").any():
print(
'Warning: "Sc" element is found in the data set, since the element is rarely found '
"in the drugs so we are not converting "
'it to single letter element, instead considering "S" '
'and "c" as separate elements. '
)
# Create a new column having processed canonical SMILES
df["processed_canonical_smiles"] = df["canonical_smiles"].copy()
# Replace the two letter elements found with one character
for pattern, repl in replacement.items():
df["processed_canonical_smiles"] = df["processed_canonical_smiles"].str.replace(
pattern, repl
)
unique_char = set(df.processed_canonical_smiles.apply(list).sum())
return df, unique_char
[12]:
# Calling function
df, unique_char = preprocessing_data(df, replace_dict)
df.head(3)
Warning: "Sc" element is found in the data set, since the element is rarely found in the drugs so we are not converting it to single letter element, instead considering "S" and "c" as separate elements.
[12]:
| chembl_id | canonical_smiles | Mol2D | processed_canonical_smiles | |
|---|---|---|---|---|
| 0 | CHEMBL207869 | Clc1c(OCc2cc(F)ccc2)ccc(Nc2c(C#Cc3ncccn3)cncn2)c1 | <rdkit.Chem.rdchem.Mol object at 0x7f824651b200> | Lc1c(OCc2cc(F)ccc2)ccc(Nc2c(C#Cc3ncccn3)cncn2)c1 |
| 1 | CHEMBL3940060 | ClCC(=O)OCCN1C(=O)Oc2c1cc1c(Nc3cc(Cl)c(F)cc3)n... | <rdkit.Chem.rdchem.Mol object at 0x7f824651b580> | LCC(=O)OCCN1C(=O)Oc2c1cc1c(Nc3cc(L)c(F)cc3)ncn... |
| 2 | CHEMBL3678951 | FC(F)(F)c1cc(Nc2n(C(C)C)c3nc(Nc4ccc(N5CC[NH+](... | <rdkit.Chem.rdchem.Mol object at 0x7f824651b6d0> | FC(F)(F)c1cc(Nc2n(C(C)C)c3nc(Nc4ccc(N5CC[NH+](... |
[13]:
# Print unique characters
print(f"All unique characters found in the preprocessed data set:\n{sorted(unique_char)}")
# NBVAL_CHECK_OUTPUT
All unique characters found in the preprocessed data set:
['#', '(', ')', '+', '-', '/', '0', '1', '2', '3', '4', '5', '6', '7', '=', '@', 'B', 'C', 'F', 'H', 'I', 'L', 'N', 'O', 'P', 'R', 'S', 'X', 'Z', '[', '\\', ']', 'c', 'n', 'o', 's']
Compute longest (& shortest) SMILES¶
Here, we compute the length and the indices in the data frame of the longest and shortest SMILES, which we will use later in the sections for visualization purpose.
[14]:
# Index of the longest SMILES string
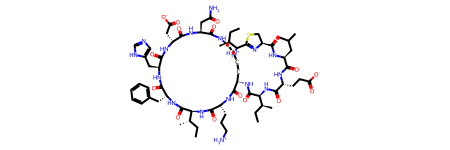
longest_smiles = max(df["canonical_smiles"], key=len)
longest_smiles_index = df.canonical_smiles[df.canonical_smiles == longest_smiles].index.tolist()
print(f"Longest SMILES: {longest_smiles}")
print(f"Contains {len(longest_smiles)} characters, index in dataframe: {longest_smiles_index[0]}.")
smiles_maxlen = len(longest_smiles)
# NBVAL_CHECK_OUTPUT
Longest SMILES: O=C(N[C@@H]1C(=O)N[C@H](CCC[NH3+])C(=O)N[C@@H]([C@H](CC)C)C(=O)N[C@H](Cc2ccccc2)C(=O)N[C@@H](Cc2[nH]cnc2)C(=O)N[C@H](CC(=O)[O-])C(=O)N[C@@H](CC(=O)N)C(=O)NCCCC1)[C@@H](NC(=O)[C@H](NC(=O)[C@@H](NC(=O)[C@H]1N=C([C@@H]([NH3+])[C@H](CC)C)SC1)CC(C)C)CCC(=O)[O-])[C@H](CC)C
Contains 267 characters, index in dataframe: 2704.
[15]:
# Index of the shortest SMILES string
shortest_smiles = min(df["canonical_smiles"], key=len)
shortest_smiles_index = df.canonical_smiles[df.canonical_smiles == shortest_smiles].index.tolist()
print(f"Shortest SMILES: {shortest_smiles}")
print(
f"Contains {len(shortest_smiles)} characters, index in dataframe: {shortest_smiles_index[0]}."
)
# NBVAL_CHECK_OUTPUT
Shortest SMILES: Oc1c(O)cccc1
Contains 12 characters, index in dataframe: 3428.
Python one-hot encoding implementation¶
One-hot encode (padding=True)¶
We define a function smiles_encoder that takes SMILES, the maximum length of the SMILES string (max_len) for padding and the list of unique characters (unique_char) present in the processed_canonical_smiles column; and returns the one-hot encoded matrix of fixed shape.
[16]:
# Function defined to create one-hot encoded matrix
def smiles_encoder(smiles, max_len, unique_char):
"""
Function defined using all unique characters in our
processed canonical SMILES structures created
with the preprocessing_data function.
Parameters
----------
smiles : str
SMILES of a molecule in string format.
unique_char : list
List of unique characters in the string data set.
max_len : int
Maximum length of the SMILES string.
Returns
-------
smiles_matrix : numpy.ndarray
One-hot encoded matrix of fixed shape
(unique char in smiles, max SMILES length).
"""
# create dictionary of the unique char data set
smi2index = {char: index for index, char in enumerate(unique_char)}
# one-hot encoding
# zero padding to max_len
smiles_matrix = np.zeros((len(unique_char), max_len))
for index, char in enumerate(smiles):
smiles_matrix[smi2index[char], index] = 1
return smiles_matrix
[17]:
# Apply the function to the processed canonical SMILES strings
df["unique_char_ohe_matrix"] = df["processed_canonical_smiles"].apply(
smiles_encoder, max_len=smiles_maxlen, unique_char=unique_char
)
df.head(3)
[17]:
| chembl_id | canonical_smiles | Mol2D | processed_canonical_smiles | unique_char_ohe_matrix | |
|---|---|---|---|---|---|
| 0 | CHEMBL207869 | Clc1c(OCc2cc(F)ccc2)ccc(Nc2c(C#Cc3ncccn3)cncn2)c1 | <rdkit.Chem.rdchem.Mol object at 0x7f824651b200> | Lc1c(OCc2cc(F)ccc2)ccc(Nc2c(C#Cc3ncccn3)cncn2)c1 | [[0.0, 0.0, 0.0, 0.0, 0.0, 0.0, 0.0, 0.0, 0.0,... |
| 1 | CHEMBL3940060 | ClCC(=O)OCCN1C(=O)Oc2c1cc1c(Nc3cc(Cl)c(F)cc3)n... | <rdkit.Chem.rdchem.Mol object at 0x7f824651b580> | LCC(=O)OCCN1C(=O)Oc2c1cc1c(Nc3cc(L)c(F)cc3)ncn... | [[0.0, 0.0, 0.0, 0.0, 0.0, 0.0, 0.0, 0.0, 0.0,... |
| 2 | CHEMBL3678951 | FC(F)(F)c1cc(Nc2n(C(C)C)c3nc(Nc4ccc(N5CC[NH+](... | <rdkit.Chem.rdchem.Mol object at 0x7f824651b6d0> | FC(F)(F)c1cc(Nc2n(C(C)C)c3nc(Nc4ccc(N5CC[NH+](... | [[0.0, 0.0, 0.0, 0.0, 0.0, 0.0, 0.0, 0.0, 0.0,... |
Visualization¶
Matplotlib is a plotting library for the python programming language and Pyplot is a state-based interface to a matplotlib module which provides a MATLAB-like interface. The imshow function in the pyplot module of the matplotlib library is used to display data as an image i.e. on a 2D space.
We now visualize our one-hot encoded matrix using imshow by defining the one_hot_matrix_plot function as shown below.
[18]:
def one_hot_matrix_plot(ohe_matrix, smiles_char, smiles):
"""
Visualize one-hot encoded matrix
using matplotlib imshow() function.
Parameters
----------
ohe_matrix : numpy.ndarray
One-hot encoded (ohe) matrix of shape
(`smiles_char`, `len(smiles)`).
smiles_char : list
List of all possible SMILES characters.
smiles : string
Original SMILES string of respective molecule.
Returns
------
None
"""
im = plt.imshow(ohe_matrix, cmap="hot", interpolation="None")
plt.xlabel("Length of SMILES string")
plt.ylabel(f"Char in SMILES ({len(smiles_char)})")
plt.title("Visualization of one-hot encoded matrix")
plt.show()
print("Shape of one-hot matrix : ", ohe_matrix.shape)
print("Associated canonical SMILES: ", smiles)
return None
[19]:
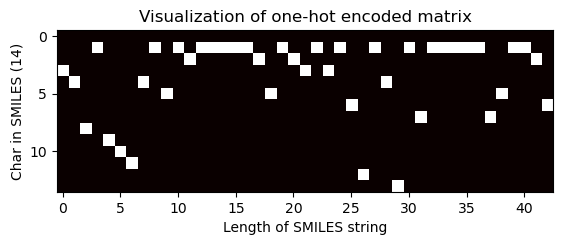
# Pass the index of the longest SMILES string to visualize the matrix
one_hot_matrix_plot(
df.iloc[longest_smiles_index[0]]["unique_char_ohe_matrix"], unique_char, longest_smiles
)
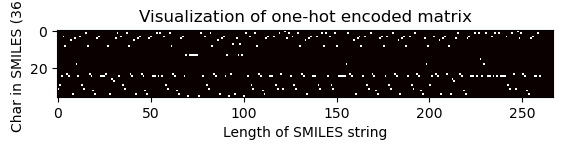
Shape of one-hot matrix : (36, 267)
Associated canonical SMILES: O=C(N[C@@H]1C(=O)N[C@H](CCC[NH3+])C(=O)N[C@@H]([C@H](CC)C)C(=O)N[C@H](Cc2ccccc2)C(=O)N[C@@H](Cc2[nH]cnc2)C(=O)N[C@H](CC(=O)[O-])C(=O)N[C@@H](CC(=O)N)C(=O)NCCCC1)[C@@H](NC(=O)[C@H](NC(=O)[C@@H](NC(=O)[C@H]1N=C([C@@H]([NH3+])[C@H](CC)C)SC1)CC(C)C)CCC(=O)[O-])[C@H](CC)C
[20]:
# Draw the molecules with the longest SMILES
longest_smiles_mol = Chem.MolFromSmiles(longest_smiles)
longest_smiles_mol
[20]:
[21]:
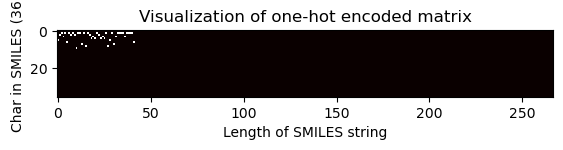
# Pass the index of the shortest SMILES string to visualize the matrix
one_hot_matrix_plot(
df.iloc[shortest_smiles_index[0]]["unique_char_ohe_matrix"], unique_char, shortest_smiles
)
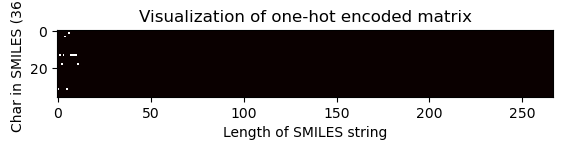
Shape of one-hot matrix : (36, 267)
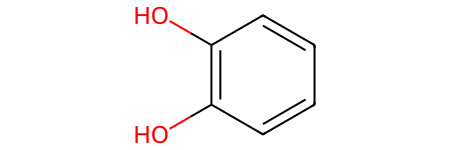
Associated canonical SMILES: Oc1c(O)cccc1
From above, the matrix visualization was performed using matplotlib imshow function, we can also visualize the entire matrix using the numpy.matrix function, e.g. the one-hot encoded matrix of the longest SMILES string as shown below.
[22]:
# Draw the molecules with the shortest SMILES
shortest_smiles_mol = Chem.MolFromSmiles(shortest_smiles)
shortest_smiles_mol
[22]:
[23]:
# Print the some rows of the matrix for the molecule with the longest smiles
np.set_printoptions(threshold=np.inf)
subset = 3
unique_char = list(unique_char)
print(
f"First {subset} rows of the ohe matrix, representing the characters {unique_char[0:subset]}\n"
)
print(np.matrix(df.iloc[longest_smiles_index[0]]["unique_char_ohe_matrix"])[0:subset, :])
First 3 rows of the ohe matrix, representing the characters ['-', ')', 'L']
[[0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0.
0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0.
0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0.
0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0.
0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0.
0. 0. 1. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0.
0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0.
0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0.
0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0.
0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0.
0. 0. 0. 0. 0. 0. 0. 1. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0.
0. 0. 0.]
[0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 1. 0. 0. 0. 0. 0. 0. 0. 0.
0. 0. 0. 0. 0. 0. 0. 0. 1. 0. 0. 0. 0. 1. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0.
0. 0. 0. 0. 0. 1. 0. 1. 0. 0. 0. 0. 1. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0.
0. 0. 0. 0. 0. 1. 0. 0. 0. 0. 1. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0.
0. 0. 0. 0. 0. 1. 0. 0. 0. 0. 1. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 1.
0. 0. 0. 0. 1. 0. 0. 0. 0. 1. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 1. 0.
1. 0. 0. 0. 0. 1. 0. 0. 0. 0. 0. 0. 1. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0.
1. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 1. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0.
1. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 1.
0. 0. 0. 0. 0. 0. 0. 0. 1. 0. 1. 0. 0. 0. 1. 0. 0. 0. 0. 1. 0. 1. 0. 0.
0. 0. 0. 0. 1. 0. 0. 0. 0. 1. 0. 0. 0. 0. 0. 0. 0. 0. 1. 0. 0. 0. 0. 0.
0. 0. 0.]
[0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0.
0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0.
0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0.
0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0.
0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0.
0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0.
0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0.
0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0.
0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0.
0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0.
0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0. 0.
0. 0. 0.]]
Discussion¶
In the fields of computer aided drug discovery and development, machine learning techniques have been used for the development of novel drug candidates. The methods for designing drug targets and novel drug discovery now oftenly combine machine learning and deep learning algorithms to enhance the efficiency, efficacy, and quality of developed outputs.
But to work with any machine learning or deep learning algorithms, the input data should be in machine readable format. In computed-aided drug design, we mainly deal with the categorical or textual data where we work with drug molecules which are represented in SMILES string format, therefore we have to convert these categorical data to a numerical format.
One-hot encoding is one of the popular and efficient encoding technique which converts the data to a numerical format. It can be used an important preprocessing steps before applying any machine learning or deep learning algorithms.
In this talktorial, we have applied one hot encoding after preprocessing the data to overcome some of the shortcomings such as:
Make equal dimension of the one-hot encoded matrices because SMILES strings could have unequal dimension since their string length might differ and for most machine learning application having equal dimension throughout the data set is required.
Replacing two character element such as
Clto a single character because while one-hot encoding, it would splitClinto two characters, namelyCandl, and that could lead to discrepancies.Looking for unique characters in the data set to produce less sparse one-hot encoded matrices.
One-hot encoding has several applications in various fields such as:
Machine learning (neural networks): In machine learning , one-hot encoding is a frequently used method to deal with categorical data because many machine learning models need their input variables to be numeric.
Natural language processing (NLP): For NLP, most of the times data consists of corpus of words which is categorical in nature. Consider we have vocabulary of size \(N\). In the one-hot encoding technique, we map the words to the vectors of length \(n\), where the \(n\)th digit is an indicator of the presence of the particular word. The \(n\)th bit of each vector indicates the presence of the \(n\)th word in the vocabulary. For example if we are converting words to the one-hot encoding format, then we will see vectors such as \([0000\dots 100], [0000\dots 010], [0000\dots 001]\), and so on. Using this technique normal sentences can be represented as vectors and numerical operations can be then performed on this vector form.
Quiz¶
Why is it required to have equal dimensions of the one-hot encoded matrix?
Is there any other way to pre-process the data?
How and which machine learning models can be applied on the above data set?
Supplementary material¶
If you are interested in other implementations of one-hot encoding, please keep reading this section. This includes:
Exploring scikit-learn and keras implementations of one-hot encoding.
Performing padding before and after one-hot encoding.
Scikit-learn implementation of one-hot encoding¶
Before implementing one-hot encoding using scikit-learn, we have defined the functions named
later_padding, which adds horizontal and vertical padding to the given matrix,and
initial_paddingwhich adds zeros to the character list after they are label encoded.
Both using the numpy.pad function as discussed in the theory section.
These functions are later used as a boolean parameter (islaterpadding and isinitialpadding) in the scikit-learn and keras implementations to choose if later padding or initial padding is required.
[24]:
# Function to add padding after one-hot encoding
def later_padding(ohe_matrix, smiles_maxlen, unique_char):
"""
Add horizontal and vertical padding
to the given matrix using numpy.pad() function.
Parameters
----------
ohe_matrix : ndarray
Character array.
smiles_max_len : int
Maximum length of the SMILES string.
unique_char : list
List of unique characters in the string data set.
Returns
-------
padded_matrix : numpy.ndarray
Padded one-hot encoded matrix of
shape (unique char in smiles, max smile_length).
"""
padded_matrix = np.pad(
ohe_matrix,
((0, smiles_maxlen - len(ohe_matrix)), (0, len(unique_char) - len(ohe_matrix[0]))),
"constant",
)
return padded_matrix
[25]:
# Function to add padding before one-hot encoding
# after label (integer) encoding
def initial_padding(smiles, max_len):
"""
Add zeroes to the list of characters
after integer encoding them
Parameters
----------
smiles : str
SMILES string.
max_len : int
Maximum length of the SMILES string
Returns
-------
canonical_char_padded : numpy.ndarray
Canonical character array padded to max_len.
"""
canonical_char = list(smiles)
# Perform padding on the list of characters
canonical_char_padded = np.pad(canonical_char, (0, max_len - len(canonical_char)), "constant")
return canonical_char_padded
One-hot encoding using scikit-learn
Now, we proceed with our second implementation of one-hot encoding from scikit-learn. We can use the OneHotEncoder from the sklearn library.
The function takes only numerical categorical values, hence any value of type string should be label_encoded first before one-hot encoding.
Thus, in the functions below first label (integer) encoded SMILES are produced, then the integer encoded SMILES are transformed to one-hot encoded matrices.
By default, the OneHotEncoder class returns a more efficient sparse encoding, which we disabled by setting the
sparse=Falseargument.
[26]:
# Use Scikit-learn implementation of one-hot encoding
def sklearn_one_hot_encoded_matrix(
smiles, islaterpadding, isinitialpadding, smiles_maxlen, unique_char
):
"""
Label and one-hot encodes the SMILES
using sklearn LabelEncoder and OneHotEncoder implementation.
Parameters
----------
smiles : str
SMILES string of a compound.
islaterpadding : bool
Paramater is `True` if `later_padding` is required,
`False` otherwise.
isinitialpadding : bool
Paramater is `True` if `initial_padding` is required,
`False` otherwise.
smile_maxlen : int
Maximum length of the SMILES string
unique_char : list
List of unique characters in the string data set.
Returns
-------
onehot_encoded : numpy.ndarray
One-hot encoded matrix of shape
(chars in individual SMILES, length of individual SMILES).
"""
# Integer encoding
canonical_char = list(smiles)
label_encoder = LabelEncoder()
# Fit_transform function is used to first fit the data and then transform it
integer_encoded = label_encoder.fit_transform(canonical_char)
# If initial padding, add zeros to vector (columns in matrix)
if isinitialpadding:
integer_encoded = initial_padding(integer_encoded, smiles_maxlen)
# One-hot encoding
onehot_encoder = OneHotEncoder(sparse=False)
# Reshape the integer encoded data
integer_encoded = integer_encoded.reshape(len(integer_encoded), 1)
onehot_encoded = onehot_encoder.fit_transform(integer_encoded)
# If later padding, add zeros to ohe matrix
if islaterpadding:
onehot_encoded = later_padding(onehot_encoded, smiles_maxlen, unique_char)
onehot_encoded = onehot_encoded.transpose()
# If initial padding, add zeros to rows
if isinitialpadding:
row_padding = np.zeros(shape=(len(unique_char) - len(onehot_encoded), smiles_maxlen))
onehot_encoded = np.append(onehot_encoded, row_padding, axis=0)
return onehot_encoded
Without padding (unequal dimension)¶
We can use the sklearn_one_hot_encoded_matrix function defined above to create the one-hot encoded matrix without padding. This will create matrices with unequal dimensions, because it will first label encode all the characters present in the SMILES strings (individually) and then one-hot encode them.
[27]:
# Apply the function over the processed canonical SMILES strings
df["sklearn_ohe_matrix_no_padding"] = df["processed_canonical_smiles"].apply(
sklearn_one_hot_encoded_matrix,
islaterpadding=False,
isinitialpadding=False,
smiles_maxlen=smiles_maxlen,
unique_char=unique_char,
)
df.head(2)
[27]:
| chembl_id | canonical_smiles | Mol2D | processed_canonical_smiles | unique_char_ohe_matrix | sklearn_ohe_matrix_no_padding | |
|---|---|---|---|---|---|---|
| 0 | CHEMBL207869 | Clc1c(OCc2cc(F)ccc2)ccc(Nc2c(C#Cc3ncccn3)cncn2)c1 | <rdkit.Chem.rdchem.Mol object at 0x7f824651b200> | Lc1c(OCc2cc(F)ccc2)ccc(Nc2c(C#Cc3ncccn3)cncn2)c1 | [[0.0, 0.0, 0.0, 0.0, 0.0, 0.0, 0.0, 0.0, 0.0,... | [[0.0, 0.0, 0.0, 0.0, 0.0, 0.0, 0.0, 0.0, 0.0,... |
| 1 | CHEMBL3940060 | ClCC(=O)OCCN1C(=O)Oc2c1cc1c(Nc3cc(Cl)c(F)cc3)n... | <rdkit.Chem.rdchem.Mol object at 0x7f824651b580> | LCC(=O)OCCN1C(=O)Oc2c1cc1c(Nc3cc(L)c(F)cc3)ncn... | [[0.0, 0.0, 0.0, 0.0, 0.0, 0.0, 0.0, 0.0, 0.0,... | [[0.0, 0.0, 0.0, 1.0, 0.0, 0.0, 0.0, 0.0, 0.0,... |
Visualization of one-hot encoded matrix (unequal dimension)
[28]:
# Choose index of the compound for which the OHE matrix is visualised
index = 100
one_hot_matrix_plot(
df.iloc[index]["sklearn_ohe_matrix_no_padding"],
df.iloc[index]["sklearn_ohe_matrix_no_padding"],
df.iloc[index]["processed_canonical_smiles"],
)
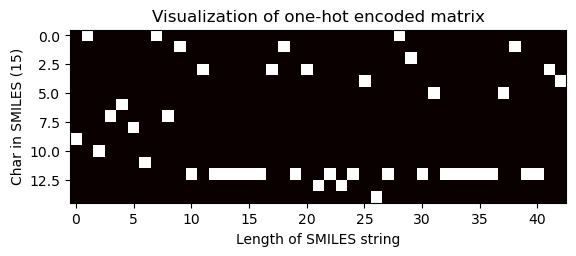
Shape of one-hot matrix : (15, 43)
Associated canonical SMILES: N([C@H](C)c1ccccc1)c1ncnc2oc(-c3ccccc3)cc12
With padding (equal dimension)¶
Padding can either be done before or after one-hot encoding is performed on the SMILES strings, meaning after we label encode the SMILES characters. We discuss both scenarios in the next sections.
Padding after one-hot encoding is performed¶
We simply pass True to the islaterpadding boolean parameter to the sklearn_one_hot_encoded_matrix function as shown below to pad the matrix after one-hot encoding is performed,
[29]:
# Apply the function over the processed canonical SMILES strings
df["sklearn_ohe_matrix_later_padding"] = df["processed_canonical_smiles"].apply(
sklearn_one_hot_encoded_matrix,
islaterpadding=True,
isinitialpadding=False,
smiles_maxlen=smiles_maxlen,
unique_char=unique_char,
)
df.head(2)
[29]:
| chembl_id | canonical_smiles | Mol2D | processed_canonical_smiles | unique_char_ohe_matrix | sklearn_ohe_matrix_no_padding | sklearn_ohe_matrix_later_padding | |
|---|---|---|---|---|---|---|---|
| 0 | CHEMBL207869 | Clc1c(OCc2cc(F)ccc2)ccc(Nc2c(C#Cc3ncccn3)cncn2)c1 | <rdkit.Chem.rdchem.Mol object at 0x7f824651b200> | Lc1c(OCc2cc(F)ccc2)ccc(Nc2c(C#Cc3ncccn3)cncn2)c1 | [[0.0, 0.0, 0.0, 0.0, 0.0, 0.0, 0.0, 0.0, 0.0,... | [[0.0, 0.0, 0.0, 0.0, 0.0, 0.0, 0.0, 0.0, 0.0,... | [[0.0, 0.0, 0.0, 0.0, 0.0, 0.0, 0.0, 0.0, 0.0,... |
| 1 | CHEMBL3940060 | ClCC(=O)OCCN1C(=O)Oc2c1cc1c(Nc3cc(Cl)c(F)cc3)n... | <rdkit.Chem.rdchem.Mol object at 0x7f824651b580> | LCC(=O)OCCN1C(=O)Oc2c1cc1c(Nc3cc(L)c(F)cc3)ncn... | [[0.0, 0.0, 0.0, 0.0, 0.0, 0.0, 0.0, 0.0, 0.0,... | [[0.0, 0.0, 0.0, 1.0, 0.0, 0.0, 0.0, 0.0, 0.0,... | [[0.0, 0.0, 0.0, 1.0, 0.0, 0.0, 0.0, 0.0, 0.0,... |
Visualization of one-hot encoded matrix (equal dimension)
[30]:
# Choose index of the compound for which the OHE matrix is visualised
index = 2705
one_hot_matrix_plot(
df.iloc[index]["sklearn_ohe_matrix_later_padding"],
unique_char,
df.iloc[index]["processed_canonical_smiles"],
)
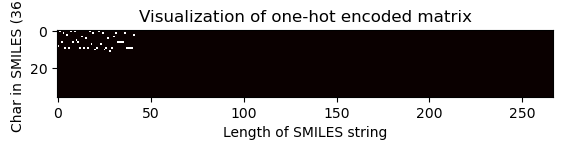
Shape of one-hot matrix : (36, 267)
Associated canonical SMILES: O(C)c1c(C(=Cc2c3c(N)nc(N)nc3oc2)CCCC)cccc1
Padding before one-hot encoding is performed¶
In this case, padding is performed before OHE - but after integer encoding - the list of SMILES characters by passing True to the initial_padding boolean parameter to the sklearn_one_hot_encoded_matrix function.
[31]:
# Apply the function over the processed canonical SMILES strings
df["sklearn_ohe_matrix_initial_padding"] = df["processed_canonical_smiles"].apply(
sklearn_one_hot_encoded_matrix,
islaterpadding=False,
isinitialpadding=True,
smiles_maxlen=smiles_maxlen,
unique_char=unique_char,
)
df.head(2)
[31]:
| chembl_id | canonical_smiles | Mol2D | processed_canonical_smiles | unique_char_ohe_matrix | sklearn_ohe_matrix_no_padding | sklearn_ohe_matrix_later_padding | sklearn_ohe_matrix_initial_padding | |
|---|---|---|---|---|---|---|---|---|
| 0 | CHEMBL207869 | Clc1c(OCc2cc(F)ccc2)ccc(Nc2c(C#Cc3ncccn3)cncn2)c1 | <rdkit.Chem.rdchem.Mol object at 0x7f824651b200> | Lc1c(OCc2cc(F)ccc2)ccc(Nc2c(C#Cc3ncccn3)cncn2)c1 | [[0.0, 0.0, 0.0, 0.0, 0.0, 0.0, 0.0, 0.0, 0.0,... | [[0.0, 0.0, 0.0, 0.0, 0.0, 0.0, 0.0, 0.0, 0.0,... | [[0.0, 0.0, 0.0, 0.0, 0.0, 0.0, 0.0, 0.0, 0.0,... | [[0.0, 0.0, 0.0, 0.0, 0.0, 0.0, 0.0, 0.0, 0.0,... |
| 1 | CHEMBL3940060 | ClCC(=O)OCCN1C(=O)Oc2c1cc1c(Nc3cc(Cl)c(F)cc3)n... | <rdkit.Chem.rdchem.Mol object at 0x7f824651b580> | LCC(=O)OCCN1C(=O)Oc2c1cc1c(Nc3cc(L)c(F)cc3)ncn... | [[0.0, 0.0, 0.0, 0.0, 0.0, 0.0, 0.0, 0.0, 0.0,... | [[0.0, 0.0, 0.0, 1.0, 0.0, 0.0, 0.0, 0.0, 0.0,... | [[0.0, 0.0, 0.0, 1.0, 0.0, 0.0, 0.0, 0.0, 0.0,... | [[0.0, 0.0, 0.0, 1.0, 0.0, 0.0, 0.0, 0.0, 0.0,... |
Visualization of one-hot encoded matrix (equal dimension)
[32]:
# Choose index of the compound for which the OHE matrix is visualised
index = 2705
one_hot_matrix_plot(
df.iloc[index]["sklearn_ohe_matrix_later_padding"],
unique_char,
df.iloc[index]["processed_canonical_smiles"],
)
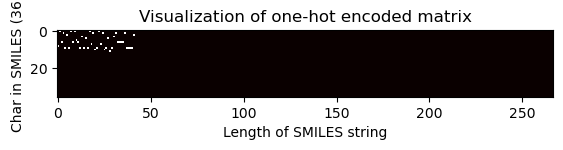
Shape of one-hot matrix : (36, 267)
Associated canonical SMILES: O(C)c1c(C(=Cc2c3c(N)nc(N)nc3oc2)CCCC)cccc1
Keras implementation of one-hot encoding¶
Keras is also a very powerful and intensively used library, especially employed in deep learning tasks. There may be a case where we have sequences or strings that are already integer encoded, then we can use the function called to_categorical(), provided by the keras library, to one-hot encode integer data directly, otherwise we can use Tokenizer to first integer encode the string data and then use the
to_categorical function to one-hot encode the data.
[33]:
# Use keras implementation of one-hot encoding
def keras_one_hot_encoded_matrix(smiles, islaterpadding, smiles_maxlen, unique_char):
"""
One-hot encodes the SMILES using keras
implementation.
Parameters
----------
canonical_char : array
Canonical character array.
islaterpadding : bool
The paramater is `True` if later_padding is required,
`False` otherwise.
smiles_maxlen : int
Maximum length of the SMILES string.
unique_char : list
List of unique characters in the string data set.
Returns
-------
encoded : numpy.ndarray
One-hot encoded matrix of shape
(chars in SMILES, length of SMILES).
"""
# Integer encoding using Tokenizer
input_smiles = smiles
tokenizer = Tokenizer(char_level=True)
tokenizer.fit_on_texts([input_smiles])
integer_encoded = tokenizer.texts_to_sequences([input_smiles])[0]
# One-hot encoding using to_categorical function
encoded = to_categorical(integer_encoded)
if islaterpadding:
encoded = later_padding(encoded, smiles_maxlen, unique_char)
encoded = encoded.transpose()
return encoded
Next, we implement two scenarios:
OHE without padding, which will result in unequal dimensions of the produced one-hot encoded matrix and
OHE with later padding by passing
Trueto the boolean parameterislaterpaddingin thekeras_one_hot_encoded_matrixfunction.
Without padding (unequal dimension)¶
[34]:
# Apply the function over the processed canonical SMILES strings
df["keras_ohe_matrix_without_padding"] = df["processed_canonical_smiles"].apply(
keras_one_hot_encoded_matrix,
smiles_maxlen=smiles_maxlen,
unique_char=unique_char,
islaterpadding=False,
)
df.head(2)
[34]:
| chembl_id | canonical_smiles | Mol2D | processed_canonical_smiles | unique_char_ohe_matrix | sklearn_ohe_matrix_no_padding | sklearn_ohe_matrix_later_padding | sklearn_ohe_matrix_initial_padding | keras_ohe_matrix_without_padding | |
|---|---|---|---|---|---|---|---|---|---|
| 0 | CHEMBL207869 | Clc1c(OCc2cc(F)ccc2)ccc(Nc2c(C#Cc3ncccn3)cncn2)c1 | <rdkit.Chem.rdchem.Mol object at 0x7f824651b200> | Lc1c(OCc2cc(F)ccc2)ccc(Nc2c(C#Cc3ncccn3)cncn2)c1 | [[0.0, 0.0, 0.0, 0.0, 0.0, 0.0, 0.0, 0.0, 0.0,... | [[0.0, 0.0, 0.0, 0.0, 0.0, 0.0, 0.0, 0.0, 0.0,... | [[0.0, 0.0, 0.0, 0.0, 0.0, 0.0, 0.0, 0.0, 0.0,... | [[0.0, 0.0, 0.0, 0.0, 0.0, 0.0, 0.0, 0.0, 0.0,... | [[0.0, 0.0, 0.0, 0.0, 0.0, 0.0, 0.0, 0.0, 0.0,... |
| 1 | CHEMBL3940060 | ClCC(=O)OCCN1C(=O)Oc2c1cc1c(Nc3cc(Cl)c(F)cc3)n... | <rdkit.Chem.rdchem.Mol object at 0x7f824651b580> | LCC(=O)OCCN1C(=O)Oc2c1cc1c(Nc3cc(L)c(F)cc3)ncn... | [[0.0, 0.0, 0.0, 0.0, 0.0, 0.0, 0.0, 0.0, 0.0,... | [[0.0, 0.0, 0.0, 1.0, 0.0, 0.0, 0.0, 0.0, 0.0,... | [[0.0, 0.0, 0.0, 1.0, 0.0, 0.0, 0.0, 0.0, 0.0,... | [[0.0, 0.0, 0.0, 1.0, 0.0, 0.0, 0.0, 0.0, 0.0,... | [[0.0, 0.0, 0.0, 0.0, 0.0, 0.0, 0.0, 0.0, 0.0,... |
Visualization of one-hot encoded matrix (unequal dimension)
[35]:
# Choose index of the compound for which the OHE matrix is visualised
index = 100
one_hot_matrix_plot(
df.iloc[index]["keras_ohe_matrix_without_padding"],
df.iloc[index]["keras_ohe_matrix_without_padding"],
df.iloc[index]["processed_canonical_smiles"],
)
Shape of one-hot matrix : (14, 43)
Associated canonical SMILES: N([C@H](C)c1ccccc1)c1ncnc2oc(-c3ccccc3)cc12
With padding (equal dimension)¶
[36]:
# Apply the function over the Processed_canonical_smiles strings
df["keras_ohe_matrix_padding"] = df["processed_canonical_smiles"].apply(
keras_one_hot_encoded_matrix,
smiles_maxlen=smiles_maxlen,
unique_char=unique_char,
islaterpadding=True,
)
df.head(2)
[36]:
| chembl_id | canonical_smiles | Mol2D | processed_canonical_smiles | unique_char_ohe_matrix | sklearn_ohe_matrix_no_padding | sklearn_ohe_matrix_later_padding | sklearn_ohe_matrix_initial_padding | keras_ohe_matrix_without_padding | keras_ohe_matrix_padding | |
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | CHEMBL207869 | Clc1c(OCc2cc(F)ccc2)ccc(Nc2c(C#Cc3ncccn3)cncn2)c1 | <rdkit.Chem.rdchem.Mol object at 0x7f824651b200> | Lc1c(OCc2cc(F)ccc2)ccc(Nc2c(C#Cc3ncccn3)cncn2)c1 | [[0.0, 0.0, 0.0, 0.0, 0.0, 0.0, 0.0, 0.0, 0.0,... | [[0.0, 0.0, 0.0, 0.0, 0.0, 0.0, 0.0, 0.0, 0.0,... | [[0.0, 0.0, 0.0, 0.0, 0.0, 0.0, 0.0, 0.0, 0.0,... | [[0.0, 0.0, 0.0, 0.0, 0.0, 0.0, 0.0, 0.0, 0.0,... | [[0.0, 0.0, 0.0, 0.0, 0.0, 0.0, 0.0, 0.0, 0.0,... | [[0.0, 0.0, 0.0, 0.0, 0.0, 0.0, 0.0, 0.0, 0.0,... |
| 1 | CHEMBL3940060 | ClCC(=O)OCCN1C(=O)Oc2c1cc1c(Nc3cc(Cl)c(F)cc3)n... | <rdkit.Chem.rdchem.Mol object at 0x7f824651b580> | LCC(=O)OCCN1C(=O)Oc2c1cc1c(Nc3cc(L)c(F)cc3)ncn... | [[0.0, 0.0, 0.0, 0.0, 0.0, 0.0, 0.0, 0.0, 0.0,... | [[0.0, 0.0, 0.0, 1.0, 0.0, 0.0, 0.0, 0.0, 0.0,... | [[0.0, 0.0, 0.0, 1.0, 0.0, 0.0, 0.0, 0.0, 0.0,... | [[0.0, 0.0, 0.0, 1.0, 0.0, 0.0, 0.0, 0.0, 0.0,... | [[0.0, 0.0, 0.0, 0.0, 0.0, 0.0, 0.0, 0.0, 0.0,... | [[0.0, 0.0, 0.0, 0.0, 0.0, 0.0, 0.0, 0.0, 0.0,... |
Visualization of one-hot encoded matrix (unequal dimension)
[37]:
# Choose index of the compound for which the OHE matrix is visualised
index = 2705
one_hot_matrix_plot(
df.iloc[index]["keras_ohe_matrix_padding"],
unique_char,
df.iloc[index]["processed_canonical_smiles"],
)
Shape of one-hot matrix : (36, 267)
Associated canonical SMILES: O(C)c1c(C(=Cc2c3c(N)nc(N)nc3oc2)CCCC)cccc1